"Physical foundations of nuclear energy. Nuclear reactor."
Topic: Physical foundations of nuclear energy. Nuclear reactor.
Lesson objectives: updating existing knowledge; continue the formation of concepts: fission of uranium nuclei, nuclear chain reaction, conditions for its occurrence, critical mass; introduce new concepts: nuclear reactor, main elements of a nuclear reactor, structure of a nuclear reactor and the principle of its operation, control of a nuclear reaction, classification of nuclear reactors and their use; continue to develop the skills to observe and draw conclusions, as well as develop the intellectual abilities and curiosity of students; continue to develop an attitude towards physics as an experimental science; cultivate a conscientious attitude to work, discipline, and a positive attitude towards knowledge.
Lesson type: learning new material.
During the classes
1. Organizational moment.
Today in the lesson we will repeat the fission of uranium nuclei, the nuclear chain reaction, the conditions for its occurrence, critical mass, we will learn what a nuclear reactor is, the main elements of a nuclear reactor, the structure of a nuclear reactor and the principle of its operation, control of a nuclear reaction, classification of nuclear reactors and their use.
2. Checking the studied material.
The mechanism of fission of uranium nuclei.
Tell us about the mechanism of a nuclear chain reaction.
Give an example of a nuclear fission reaction of a uranium nucleus.
What is called critical mass?
How does a chain reaction occur in uranium if its mass is less than critical or greater than critical?
What is the critical mass of uranium 295? Is it possible to reduce the critical mass?
In what ways can you change the course of a nuclear chain reaction?
What is the purpose of slowing down fast neutrons?
What substances are used as moderators?
3. Explanation of new material.
: What is the main part of any nuclear power plant? ( nuclear reactor)
Well done. So, guys, now let’s look at this issue in more detail.
Historical reference.
Igor Vasilyevich Kurchatov is an outstanding Soviet physicist, academician, founder and first director of the Institute of Atomic Energy from 1943 to 1960, chief scientific director of the atomic problem in the USSR, one of the founders of the use of nuclear energy for peaceful purposes. Academician of the USSR Academy of Sciences (1943). The first Soviet atomic bomb was tested in 1949. Four years later, the world's first hydrogen bomb was successfully tested. And in 1949, Igor Vasilyevich Kurchatov began work on a nuclear power plant project. Nuclear power plant is the herald of the peaceful use of atomic energy. The project was successfully completed: on July 27, 1954, our nuclear power plant became the first in the world! Kurchatov rejoiced and had fun like a child!
Definition of a nuclear reactor.
A nuclear reactor is a device in which a controlled chain reaction of fission of certain heavy nuclei is carried out and maintained.
The first nuclear reactor was built in 1942 in the USA under the leadership of E. Fermi. In our country, the first reactor was built in 1946 under the leadership of I.V. Kurchatov.
The main elements of a nuclear reactor are:
nuclear fuel (uranium 235, uranium 238, plutonium 239);
neutron moderator (heavy water, graphite, etc.);
coolant for removing the energy generated during reactor operation (water, liquid sodium, etc.);
Control rods (boron, cadmium) - highly absorbing neutrons
A protective shell that blocks radiation (concrete with iron filler).
Operating principle nuclear reactor
Nuclear fuel is located in the core in the form of vertical rods called fuel elements (fuel elements). Fuel rods are designed to regulate reactor power.
The mass of each fuel rod is significantly less than the critical mass, so a chain reaction cannot occur in one rod. It begins after all uranium rods are immersed in the core.
The core is surrounded by a layer of substance that reflects neutrons (reflector) and a protective shell of concrete that traps neutrons and other particles.
Heat removal from fuel cells. The coolant, water, washes the rod, heated to 300°C at high pressure, and enters the heat exchangers.
The role of the heat exchanger is that water heated to 300°C gives off heat to ordinary water and turns into steam.
Nuclear Reaction Control
The reactor is controlled using rods containing cadmium or boron. When the rods are extended from the reactor core, K > 1, and when fully retracted - K< 1. Вдвигая стержни внутрь активной зоны, можно в любой момент времени приостановить развитие цепной реакции. Управление ядерными реакторами осуществляется дистанционно с помощью ЭВМ.
Slow neutron reactor.
The most efficient fission of uranium-235 nuclei occurs under the influence of slow neutrons. Such reactors are called slow neutron reactors. Secondary neutrons produced by a fission reaction are fast. In order for their subsequent interaction with uranium-235 nuclei in the chain reaction to be most effective, they are slowed down by introducing a moderator into the core - a substance that reduces the kinetic energy of neutrons.
Fast neutron reactor.
Fast neutron reactors cannot operate on natural uranium. The reaction can only be maintained in an enriched mixture containing at least 15% uranium isotope. The advantage of fast neutron reactors is that their operation produces a significant amount of plutonium, which can then be used as nuclear fuel.
Homogeneous and heterogeneous reactors.
Nuclear reactors, depending on the relative placement of fuel and moderator, are divided into homogeneous and heterogeneous. In a homogeneous reactor, the core is a homogeneous mass of fuel, moderator and coolant in the form of a solution, mixture or melt. A reactor in which fuel in the form of blocks or fuel assemblies is placed in a moderator, forming a regular geometric lattice in it, is called heterogeneous.
Conversion of internal energy of atomic nuclei into electrical energy.
A nuclear reactor is the main element of a nuclear power plant (NPP), which converts thermal nuclear energy into electrical energy. Energy conversion occurs according to the following scheme:
internal energy of uranium nuclei -
kinetic energy of neutrons and nuclear fragments -
internal energy of water -
internal energy of steam -
kinetic energy of steam -
kinetic energy of the turbine rotor and generator rotor -
Electric Energy.
Use of nuclear reactors.
Depending on their purpose, nuclear reactors can be power reactors, converters and breeders, research and multipurpose, transport and industrial.
Nuclear power reactors are used to generate electricity in nuclear power plants, ship power plants, nuclear combined heat and power plants, and nuclear heat supply stations.
Reactors designed to produce secondary nuclear fuel from natural uranium and thorium are called converters or breeders. In the converter reactor, secondary nuclear fuel produces less than what was initially consumed.
In a breeder reactor, expanded reproduction of nuclear fuel is carried out, i.e. it turns out more than was spent.
Research reactors are used to study the processes of interaction of neutrons with matter, study the behavior of reactor materials in intense fields of neutron and gamma radiation, radiochemical and biological research, the production of isotopes, and experimental research into the physics of nuclear reactors.
Reactors have different powers, stationary or pulsed operating modes. Multipurpose reactors are those that serve several purposes, such as generating energy and producing nuclear fuel.
Environmental disasters at nuclear power plants
1957 – accident in Great Britain
1966 – partial meltdown of the core after a reactor cooling failure near Detroit.
1971 - a lot of polluted water went into the US River
1979 - the largest accident in the USA
1982 – release of radioactive steam into the atmosphere
1983 - a terrible accident in Canada (radioactive water flowed out for 20 minutes - a ton per minute)
1986 – accident in Great Britain
1986 – accident in Germany
1986 – Chernobyl Nuclear Power Plant
1988 – fire at a nuclear power plant in Japan
Modern nuclear power plants are equipped with PCs, but previously, even after an accident, reactors continued to operate, since there was no automatic shutdown system.
4. Fixing the material.
What is a nuclear reactor called?
What is the nuclear fuel in a reactor?
What substance serves as a neutron moderator in a nuclear reactor?
What is the purpose of a neutron moderator?
What are control rods used for? How are they used?
What is used as coolant in nuclear reactors?
Why is it necessary for the mass of each uranium rod to be less than the critical mass?
5. Test execution.
What particles are involved in the fission of uranium nuclei?
A. protons;
B. neutrons;
B. electrons;
G. helium nuclei.
What mass of uranium is critical?
A. the greatest at which a chain reaction is possible;
B. any mass;
B. the smallest at which a chain reaction is possible;
D. the mass at which the reaction will stop.
What is the approximate critical mass of uranium 235?
A. 9 kg;
B. 20 kg;
B. 50 kg;
G. 90 kg.
Which of the following substances can be used in nuclear reactors as neutron moderators?
A. graphite;
B. cadmium;
B. heavy water;
G. boron.
For a nuclear chain reaction to occur at a nuclear power plant, the neutron multiplication factor must be:
A. is equal to 1;
B. more than 1;
V. less than 1.
The rate of fission of heavy atom nuclei in nuclear reactors is controlled by:
A. due to the absorption of neutrons when lowering rods with an absorber;
B. due to an increase in heat removal with an increase in coolant speed;
B. by increasing the supply of electricity to consumers;
G. by reducing the mass of nuclear fuel in the core when removing rods with fuel.
What energy transformations occur in a nuclear reactor?
A. the internal energy of atomic nuclei is converted into light energy;
B. the internal energy of atomic nuclei is converted into mechanical energy;
B. the internal energy of atomic nuclei is converted into electrical energy;
D. none of the answers is correct.
In 1946, the first nuclear reactor was built in the Soviet Union. Who was the leader of this project?
A. S. Korolev;
B. I. Kurchatov;
V. D. Sakharov;
G. A. Prokhorov.
Which way do you consider the most acceptable for increasing the reliability of nuclear power plants and preventing contamination of the external environment?
A. development of reactors capable of automatically cooling the reactor core regardless of the will of the operator;
B. increasing the literacy of NPP operation, the level of professional preparedness of NPP operators;
B. development of highly efficient technologies for dismantling nuclear power plants and processing radioactive waste;
D. location of reactors deep underground;
D. refusal to build and operate a nuclear power plant.
What sources of environmental pollution are associated with the operation of nuclear power plants?
A. uranium industry;
B. nuclear reactors of various types;
B. radiochemical industry;
D. sites for processing and disposal of radioactive waste;
D. use of radionuclides in the national economy; E. nuclear explosions.
Answers: 1 B; 2 V; 3 V; 4 A, B; 5 A; 6 A; 7 V;. 8 B; 9 B.V; 10 A, B, C, D, E.
6. Lesson summary.
What new did you learn in class today?
What did you like about the lesson?
What questions do you have?
A fission chain reaction is always accompanied by the release of enormous energy. The practical use of this energy is the main task of a nuclear reactor.
A nuclear reactor is a device in which a controlled, or controlled, nuclear fission reaction occurs.
Based on the principle of operation, nuclear reactors are divided into two groups: thermal neutron reactors and fast neutron reactors.
How does a thermal neutron nuclear reactor work?
A typical nuclear reactor has:
- Core and moderator;
- Neutron reflector;
- Coolant;
- Chain reaction control system, emergency protection;
- Control and radiation protection system;
- Remote control system.

1 - active zone; 2 - reflector; 3 - protection; 4 - control rods; 5 - coolant; 6 - pumps; 7 - heat exchanger; 8 - turbine; 9 - generator; 10 - capacitor.
Core and moderator
It is in the core that a controlled fission chain reaction occurs.
Most nuclear reactors operate on heavy isotopes of uranium-235. But in natural samples of uranium ore its content is only 0.72%. This concentration is not enough for a chain reaction to develop. Therefore, the ore is artificially enriched, bringing the content of this isotope to 3%.
Fissile material, or nuclear fuel, in the form of tablets is placed in hermetically sealed rods, which are called fuel rods (fuel elements). They permeate the entire active zone filled with moderator neutrons.
Why is a neutron moderator needed in a nuclear reactor?
The fact is that the neutrons born after the decay of uranium-235 nuclei have a very high speed. The probability of their capture by other uranium nuclei is hundreds of times less than the probability of capture of slow neutrons. And if their speed is not reduced, the nuclear reaction may die out over time. The moderator solves the problem of reducing the speed of neutrons. If water or graphite is placed in the path of fast neutrons, their speed can be artificially reduced and thus the number of particles captured by atoms can be increased. At the same time, a chain reaction in the reactor will require less nuclear fuel.
As a result of the slowdown process, thermal neutrons, the speed of which is almost equal to the speed of thermal movement of gas molecules at room temperature.
Water, heavy water (deuterium oxide D 2 O), beryllium, and graphite are used as a moderator in nuclear reactors. But the best moderator is heavy water D2O.
Neutron reflector
To avoid neutron leakage into the environment, the core of a nuclear reactor is surrounded by neutron reflector. The material used for reflectors is often the same as in moderators.
Coolant
The heat released during a nuclear reaction is removed using a coolant. Ordinary natural water, previously purified from various impurities and gases, is often used as a coolant in nuclear reactors. But since water boils already at a temperature of 100 0 C and a pressure of 1 atm, in order to increase the boiling point, the pressure in the primary coolant circuit is increased. The primary circuit water circulating through the reactor core washes the fuel rods, heating up to a temperature of 320 0 C. Then, inside the heat exchanger, it gives off heat to the secondary circuit water. The exchange takes place through heat exchange tubes, so there is no contact with the secondary circuit water. This prevents radioactive substances from entering the second circuit of the heat exchanger.
And then everything happens as at a thermal power plant. Water in the second circuit turns into steam. The steam rotates a turbine, which drives an electric generator, which produces electric current.
In heavy water reactors, the coolant is heavy water D2O, and in reactors with liquid metal coolants it is molten metal.
Chain reaction control system
The current state of the reactor is characterized by a quantity called reactivity.
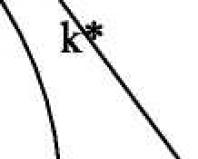
ρ = ( k -1)/ k ,
k = n i / n i -1 ,
Where k – neutron multiplication factor,
n i - the number of neutrons of the next generation in the nuclear fission reaction,
n i -1 , - the number of neutrons of the previous generation in the same reaction.
If k ˃ 1 , the chain reaction grows, the system is called supercritical y. If k< 1 , the chain reaction dies out, and the system is called subcritical. At k = 1 the reactor is in stable critical condition, since the number of fissile nuclei does not change. In this state reactivity ρ = 0 .
The critical state of the reactor (the required neutron multiplication factor in a nuclear reactor) is maintained by moving control rods. The material from which they are made includes neutron absorbent substances. By extending or pushing these rods into the core, the rate of the nuclear fission reaction is controlled.
The control system provides control of the reactor during its startup, scheduled shutdown, operation at power, as well as emergency protection of the nuclear reactor. This is achieved by changing the position of the control rods.
If any of the reactor parameters (temperature, pressure, rate of power rise, fuel consumption, etc.) deviates from the norm, and this can lead to an accident, special emergency rods and the nuclear reaction quickly stops.
Ensure that the reactor parameters comply with the standards control and radiation protection systems.
To protect the environment from radioactive radiation, the reactor is placed in a thick concrete shell.
Remote control systems
All signals about the state of the nuclear reactor (coolant temperature, radiation level in different parts of the reactor, etc.) are sent to the reactor control panel and processed in computer systems. The operator receives all the necessary information and recommendations for eliminating certain deviations.
Fast reactors

The difference between reactors of this type and thermal neutron reactors is that fast neutrons arising after the decay of uranium-235 are not slowed down, but are absorbed by uranium-238 with its subsequent conversion into plutonium-239. Therefore, fast neutron reactors are used to produce weapons-grade plutonium-239 and thermal energy, which nuclear power plant generators convert into electrical energy.
The nuclear fuel in such reactors is uranium-238, and the raw material is uranium-235.
In natural uranium ore, 99.2745% is uranium-238. When a thermal neutron is absorbed, it does not fission, but becomes an isotope of uranium-239.
Some time after β-decay, uranium-239 turns into a neptunium-239 nucleus:
239 92 U → 239 93 Np + 0 -1 e
After the second β-decay, fissile plutonium-239 is formed:
239 9 3 Np → 239 94 Pu + 0 -1 e
And finally, after the alpha decay of the plutonium-239 nucleus, uranium-235 is obtained:
239 94 Pu → 235 92 U + 4 2 He
Fuel rods with raw materials (enriched uranium-235) are located in the reactor core. This zone is surrounded by a breeding zone, which consists of fuel rods with fuel (depleted uranium-238). Fast neutrons emitted from the core after the decay of uranium-235 are captured by uranium-238 nuclei. As a result, plutonium-239 is formed. Thus, new nuclear fuel is produced in fast neutron reactors.
Liquid metals or mixtures thereof are used as coolants in fast neutron nuclear reactors.
Classification and application of nuclear reactors

Nuclear reactors are mainly used in nuclear power plants. With their help, electrical and thermal energy is produced on an industrial scale. Such reactors are called energy .
Nuclear reactors are widely used in the propulsion systems of modern nuclear submarines, surface ships, and in space technology. They supply motors with electrical energy and are called transport reactors .
For scientific research in the field of nuclear physics and radiation chemistry, fluxes of neutrons and gamma quanta are used, which are obtained in the core research reactors. The energy generated by them does not exceed 100 MW and is not used for industrial purposes.
Power experimental reactors even less. It reaches a value of only a few kW. These reactors study various physical quantities, the meaning of which is important in the design of nuclear reactions.
TO industrial reactors include reactors for the production of radioactive isotopes used for medical purposes, as well as in various fields of industry and technology. Seawater desalination reactors are also classified as industrial reactors.
The nuclear reactor works smoothly and efficiently. Otherwise, as you know, there will be trouble. But what's going on inside? Let's try to formulate the principle of operation of a nuclear (nuclear) reactor briefly, clearly, with stops.
In essence, the same process is happening there as during a nuclear explosion. Only the explosion happens very quickly, but in the reactor all this stretches out for a long time. As a result, everything remains safe and sound, and we receive energy. Not so much that everything around would be destroyed at once, but quite sufficient to provide electricity to the city.

Before you understand how a controlled nuclear reaction occurs, you need to know what it is. nuclear reaction at all.
Nuclear reaction is the process of transformation (fission) of atomic nuclei when they interact with elementary particles and gamma quanta.
Nuclear reactions can occur with both absorption and release of energy. The reactor uses the second reactions.
Nuclear reactor is a device whose purpose is to maintain a controlled nuclear reaction with the release of energy.
Often a nuclear reactor is also called an atomic reactor. Let us note that there is no fundamental difference here, but from the point of view of science it is more correct to use the word “nuclear”. There are now many types of nuclear reactors. These are huge industrial reactors designed to generate energy in power plants, nuclear reactors of submarines, small experimental reactors used in scientific experiments. There are even reactors used to desalinate seawater.

The history of the creation of a nuclear reactor
The first nuclear reactor was launched in the not-so-distant 1942. This happened in the USA under the leadership of Fermi. This reactor was called the "Chicago Woodpile".
In 1946, the first Soviet reactor, launched under the leadership of Kurchatov, began operating. The body of this reactor was a ball of seven meters in diameter. The first reactors did not have a cooling system, and their power was minimal. By the way, the Soviet reactor had an average power of 20 Watts, and the American one - only 1 Watt. For comparison, the average power of modern power reactors is 5 Gigawatts. Less than ten years after the launch of the first reactor, the world's first industrial nuclear power plant was opened in the city of Obninsk.

The principle of operation of a nuclear (nuclear) reactor
Any nuclear reactor has several parts: core With fuel And moderator , neutron reflector , coolant , control and protection system . Isotopes are most often used as fuel in reactors. uranium (235, 238, 233), plutonium (239) and thorium (232). The core is a boiler through which ordinary water (coolant) flows. Among other coolants, “heavy water” and liquid graphite are less commonly used. If we talk about the operation of nuclear power plants, then a nuclear reactor is used to produce heat. Electricity itself is generated using the same method as in other types of power plants - steam rotates a turbine, and the energy of movement is converted into electrical energy.
Below is a diagram of the operation of a nuclear reactor.

As we have already said, the decay of a heavy uranium nucleus produces lighter elements and several neutrons. The resulting neutrons collide with other nuclei, also causing them to fission. At the same time, the number of neutrons grows like an avalanche.
It should be mentioned here neutron multiplication factor . So, if this coefficient exceeds a value equal to one, a nuclear explosion occurs. If the value is less than one, there are too few neutrons and the reaction dies out. But if you maintain the value of the coefficient equal to one, the reaction will proceed long and stably.

The question is how to do this? In the reactor, the fuel is in the so-called fuel elements (TVELakh). These are rods that contain, in the form of small tablets, nuclear fuel . Fuel rods are connected into hexagonal-shaped cassettes, of which there can be hundreds in a reactor. Cassettes with fuel rods are arranged vertically, and each fuel rod has a system that allows you to adjust the depth of its immersion into the core. In addition to the cassettes themselves, they include control rods And emergency protection rods . The rods are made of a material that absorbs neutrons well. Thus, control rods can be lowered to different depths in the core, thereby adjusting the neutron multiplication factor. Emergency rods are designed to shut down the reactor in case of an emergency.

How is a nuclear reactor started?
We have figured out the operating principle itself, but how to start and make the reactor function? Roughly speaking, here it is - a piece of uranium, but the chain reaction does not begin in it on its own. The fact is that in nuclear physics there is a concept critical mass .

Critical mass is the mass of fissile material required to start a nuclear chain reaction.
With the help of fuel rods and control rods, a critical mass of nuclear fuel is first created in the reactor, and then the reactor is brought to the optimal power level in several stages.
In this article, we tried to give you a general idea of the structure and operating principle of a nuclear (nuclear) reactor. If you have any questions on the topic or have been asked a problem in nuclear physics at the university, please contact to the specialists of our company. As usual, we are ready to help you resolve any pressing issue regarding your studies. And while we're at it, here's another educational video for your attention!
Nuclear reactor
A nuclear reactor is a device in which a controlled nuclear chain reaction occurs, accompanied by the release of energy. The first nuclear reactor was built and launched in December 1942 in the USA under the leadership of E. Fermi. The first reactor built outside the United States was ZEEP, launched in Canada in September 1945. In Europe, the first nuclear reactor was the F-1 installation, which started operating on December 25, 1946 in Moscow under the leadership of I.V. Kurchatov.
By 1978, there were already about a hundred nuclear reactors of various types operating in the world. The components of any nuclear reactor are: a core with nuclear fuel, usually surrounded by a neutron reflector, a coolant, a chain reaction control system, radiation protection, and a remote control system. The reactor vessel is subject to wear (especially under the influence of ionizing radiation). The main characteristic of a nuclear reactor is its power. A power of 1 MW corresponds to a chain reaction in which 3·1016 fission events occur in 1 second.
Story

The theoretical group “Uranium Project” of Nazi Germany, working in the Kaiser Wilhelm Society, was headed by Weizsäcker, but only formally. Heisenberg became the de facto leader, developing the theoretical foundations of the chain reaction, while Weizsäcker and a group of participants focused on creating a “uranium machine” - the first reactor. In the late spring of 1940, one of the group's scientists, Harteck, conducted the first experiment attempting to create a chain reaction using uranium oxide and a solid graphite moderator. However, the available fissile material was not sufficient to achieve this goal. In 1941, at the University of Leipzig, a member of Heisenberg's group, Doepel, built a stand with a heavy water moderator, in experiments on which, by May 1942, it was possible to achieve the production of neutrons in quantities exceeding their absorption. German scientists managed to achieve a full-fledged chain reaction in February 1945 in an experiment conducted in a mine working near Haigerloch. However, a few weeks later, Germany's nuclear program ceased to exist.
The nuclear fission chain reaction (chain reaction for short) was first carried out in December 1942. A group of physicists at the University of Chicago, led by E. Fermi, created the world's first nuclear reactor, called the Chicago Pile-1 (CP-1). It consisted of graphite blocks, between which were located balls of natural uranium and its dioxide. Fast neutrons appearing after the fission of 235U nuclei were slowed down by graphite to thermal energies, and then caused new nuclear fissions. Reactors like SR-1, in which the majority of fissions occur under the influence of thermal neutrons, are called thermal neutron reactors. They contain a lot of moderator compared to nuclear fuel.
In the USSR, theoretical and experimental studies of the features of startup, operation and control of reactors were carried out by a group of physicists and engineers under the leadership of Academician I.V. Kurchatov. The first Soviet reactor F-1 was built in Laboratory No. 2 of the USSR Academy of Sciences (Moscow). This reactor was brought into critical condition on December 25, 1946. The F-1 reactor was made of graphite blocks and had the shape of a ball with a diameter of approximately 7.5 m. In the central part of the ball with a diameter of 6 m, uranium rods were placed through holes in the graphite blocks. The F-1 reactor, like the CP-1 reactor, did not have a cooling system, so it operated at very low power levels (fractions of a watt, rarely a few watts). The results of research at the F-1 reactor became the basis for projects of more complex industrial reactors. In 1948, the I-1 reactor (according to other sources, it was called A-1) for the production of plutonium was put into operation, and on June 27, 1954, the world's first nuclear power plant with an electrical capacity of 5 MW in Obninsk came into operation.
Design and principle of operation
Energy release mechanism The transformation of a substance is accompanied by the release of free energy only if the substance has a reserve of energy. The latter means that microparticles of a substance are in a state with a rest energy greater than in another possible state to which a transition exists. A spontaneous transition is always prevented by an energy barrier, to overcome which the microparticle must receive a certain amount of energy from the outside - excitation energy. The exoenergetic reaction consists in the fact that in the transformation following excitation, more energy is released than is required to excite the process. There are two ways to overcome the energy barrier: either due to the kinetic energy of colliding particles, or due to the binding energy of the joining particle.
If we keep in mind the macroscopic scale of energy release, then all or initially at least some fraction of particles of the substance must have the kinetic energy necessary to excite reactions. This is achievable only by increasing the temperature of the medium to a value at which the energy of thermal motion approaches the energy threshold limiting the course of the process. In the case of molecular transformations, that is, chemical reactions, such an increase is usually hundreds of kelvins, but in the case of nuclear reactions it is at least 107 K due to the very high height of the Coulomb barriers of colliding nuclei. Thermal excitation of nuclear reactions is carried out in practice only during the synthesis of the lightest nuclei, in which the Coulomb barriers are minimal (thermonuclear fusion).
Excitation by joining particles does not require large kinetic energy, and, therefore, does not depend on the temperature of the medium, since it occurs due to unused bonds inherent in the attractive forces of particles. But to excite reactions, the particles themselves are necessary. And if we again mean not a separate act of reaction, but the production of energy on a macroscopic scale, then this is possible only when a chain reaction occurs. The latter occurs when the particles that excite the reaction reappear as products of an exoenergetic reaction.
Design
Any nuclear reactor consists of the following parts:
- Core with nuclear fuel and moderator;
- Neutron reflector surrounding the core;
- Coolant;
- Chain reaction control system, including emergency protection;
- Radiation protection;
- Remote control system.
Iodine pit
Iodine pit is a state of a nuclear reactor after it is turned off, characterized by the accumulation of the short-lived xenon isotope 135Xe. This process leads to the temporary appearance of significant negative reactivity, which, in turn, makes it impossible to bring the reactor to its design capacity within a certain period (about 1-2 days).
Classification
By purpose
According to the nature of their use, nuclear reactors are divided into:
- Energy reactors designed to produce electrical and thermal energy used in the energy sector, as well as for desalination of sea water (desalination reactors are also classified as industrial). Such reactors are mainly used in nuclear power plants. The thermal power of modern power reactors reaches 5 GW. A separate group includes:
- Transport reactors designed to supply energy to vehicle engines. The widest groups of applications are marine transport reactors used on submarines and various surface vessels, as well as reactors used in space technology.
- Experimental reactors designed to study various physical quantities whose significance is necessary for the design and operation of nuclear reactors; The power of such reactors does not exceed several kW.
- Research reactors, in which fluxes of neutrons and gamma quanta created in the core are used for research in the field of nuclear physics, solid state physics, radiation chemistry, biology, and for testing materials intended to operate in intense neutron fluxes (including . parts of nuclear reactors), for the production of isotopes. The power of research reactors does not exceed 100 MW. The released energy is usually not used.
- Industrial (weapons, isotope) reactors used to produce isotopes used in various fields. Most widely used for the production of nuclear weapons materials, such as 239Pu. Also classified as industrial are reactors used for desalination of sea water.
Reactors are often used to solve two or more different problems, in which case they are called multi-purpose. For example, some power reactors, especially in the early days of nuclear power, were designed primarily for experimentation. Fast neutron reactors can simultaneously produce energy and produce isotopes. Industrial reactors, in addition to their main task, often generate electrical and thermal energy.
According to the neutron spectrum
- Thermal (slow) neutron reactor (“thermal reactor”)
- Fast neutron reactor ("fast reactor")
- Intermediate Neutron Reactor
- Mixed Spectrum Reactor
By fuel placement
- Heterogeneous reactors, where fuel is placed discretely in the core in the form of blocks, between which there is a moderator;
- Homogeneous reactors, where the fuel and moderator are a homogeneous mixture (homogeneous system).
In a heterogeneous reactor, the fuel and moderator can be spatially separated, in particular, in a cavity reactor, the moderator-reflector surrounds a cavity with fuel that does not contain a moderator. From a nuclear physical point of view, the criterion for homogeneity/heterogeneity is not the design, but the placement of fuel blocks at a distance exceeding the neutron moderation length in a given moderator. Thus, reactors with the so-called “close lattice” are designed as homogeneous, although in them the fuel is usually separated from the moderator.
Nuclear fuel blocks in a heterogeneous reactor are called fuel assemblies (FA), which are placed in the core at the nodes of a regular lattice, forming cells.
By fuel type
- uranium isotopes 235, 238, 233 (235U, 238U, 233U)
- plutonium isotope 239 (239Pu), also isotopes 239-242Pu in the form of a mixture with 238U (MOX fuel)
- thorium isotope 232 (232Th) (via conversion to 233U)
By degree of enrichment:
- natural uranium
- weakly enriched uranium
- highly enriched uranium
By chemical composition:
- metal U
- UO2 (uranium dioxide)
- UC (uranium carbide), etc.
By type of coolant
- H2O (Water-Water Reactor)
- Gas, (Graphite-gas reactor)
- Organic cooled reactor
- Liquid metal cooled reactor
- Molten salt reactor
- Solid coolant reactor
By type of moderator
- C (Graphite-gas reactor, Graphite-water reactor)
- H2O (Light water reactor, Water-cooled reactor, VVER)
- D2O (Heavy water nuclear reactor, CANDU)
- Be, BeO
- Metal hydrides
- Without moderator (Fast reactor)
By design
- Vessel reactors
- Channel reactors
By steam generation method
- Reactor with external steam generator (water-water reactor, VVER)
- Boiling Reactor
IAEA classification
- PWR (pressurized water reactors) - water-water reactor (pressurized water reactor);
- BWR (boiling water reactor) - boiling water reactor;
- FBR (fast breeder reactor) - fast breeder reactor;
- GCR (gas-cooled reactor) - gas-cooled reactor;
- LWGR (light water graphite reactor) - graphite-water reactor
- PHWR (pressurized heavy water reactor) - heavy water reactor
The most common in the world are pressurized water (about 62%) and boiling water (20%) reactors.
Nuclear reactor control
Control of a nuclear reactor is possible only due to the fact that during fission, some of the neutrons fly out of the fragments with a delay, which can range from several milliseconds to several minutes.
To control the reactor, absorber rods are used, introduced into the core, made of materials that strongly absorb neutrons (mainly B, Cd and some others) and/or a solution of boric acid added to the coolant in a certain concentration (boron control). The movement of the rods is controlled by special mechanisms, drives, operating according to signals from the operator or equipment for automatic control of the neutron flux.
In case of various emergency situations, each reactor is provided with an emergency termination of the chain reaction, carried out by dropping all absorbing rods into the core - an emergency protection system.
Residual Heat
An important issue directly related to nuclear safety is decay heat. This is a specific feature of nuclear fuel, which consists in the fact that, after the cessation of the fission chain reaction and the thermal inertia usual for any energy source, the release of heat in the reactor continues for a long time, which creates a number of technically complex problems.
Residual heat release is a consequence of the β- and γ-decay of fission products that accumulated in the fuel during reactor operation. Fission product nuclei, due to decay, transform into a more stable or completely stable state with the release of significant energy.
Although the decay heat release rate quickly decreases to values small compared to steady-state values, in high-power power reactors it is significant in absolute terms. For this reason, residual heat generation entails the need for a long period of time to ensure heat removal from the reactor core after it is shut down. This task requires the design of the reactor installation to have cooling systems with a reliable power supply, and also necessitates long-term (3-4 years) storage of spent nuclear fuel in storage facilities with a special temperature regime - cooling pools, which are usually located in close proximity to the reactor.
Nuclear reactors have one job: to split atoms in a controlled reaction and use the released energy to generate electrical power. For many years, reactors were seen as both a miracle and a threat.
When the first commercial U.S. reactor came online at Shippingport, Pennsylvania, in 1956, the technology was hailed as the energy source of the future, and some believed the reactors would make generating electricity too cheap. There are now 442 nuclear reactors built worldwide, about a quarter of these reactors are in the United States. The world has become dependent on nuclear reactors, producing 14 percent of its electricity. Futurists even fantasized about nuclear cars.
When the Unit 2 reactor at the Three Mile Island Power Plant in Pennsylvania experienced a cooling system failure and partial meltdown of its radioactive fuel in 1979, the warm feelings about reactors changed radically. Even though the destroyed reactor was contained and no serious radiation emitted, many people began to view the reactors as too complex and vulnerable, with potentially catastrophic consequences. People were also concerned about radioactive waste from the reactors. As a result, construction of new nuclear power plants in the United States has stalled. When a more serious accident occurred at the Chernobyl nuclear power plant in the Soviet Union in 1986, nuclear power seemed doomed.
But in the early 2000s, nuclear reactors began to make a comeback, thanks to rising energy demands and dwindling supplies of fossil fuels, as well as growing concerns about climate change resulting from carbon dioxide emissions.
But in March 2011, another crisis occurred - this time the Fukushima 1 nuclear power plant in Japan was badly damaged by an earthquake.
Use of nuclear reaction
Simply put, a nuclear reactor splits atoms and releases the energy that holds their parts together.
If you've forgotten high school physics, we'll remind you how nuclear fission works. Atoms are like tiny solar systems, with a core like the Sun and electrons like planets in orbit around it. The nucleus is made up of particles called protons and neutrons, which are bound together. The force that binds the elements of the core is difficult to even imagine. It is many billions of times stronger than the force of gravity. Despite this enormous force, it is possible to split a nucleus—by shooting neutrons at it. When this is done, a lot of energy will be released. When atoms decay, their particles crash into nearby atoms, splitting them, and those, in turn, are next, and next, and next. There is a so-called chain reaction.
Uranium, an element with large atoms, is ideal for the fission process because the force that binds the particles of its nucleus is relatively weak compared to other elements. Nuclear reactors use a specific isotope called Uran-235 . Uranium-235 is rare in nature, with ore from uranium mines containing only about 0.7% Uranium-235. This is why reactors are used enrichedUwounds, which is created by separating and concentrating Uranium-235 through a gas diffusion process.

A chain reaction process can be created in an atomic bomb, similar to those dropped on the Japanese cities of Hiroshima and Nagasaki during World War II. But in a nuclear reactor, the chain reaction is controlled by inserting control rods made of materials such as cadmium, hafnium or boron that absorb some of the neutrons. This still allows the fission process to release enough energy to heat the water to about 270 degrees Celsius and turn it into steam, which is used to spin the power plant's turbines and generate electricity. Basically, in this case, a controlled nuclear bomb works instead of coal to create electricity, except that the energy to boil the water comes from splitting atoms instead of burning carbon.
Nuclear Reactor Components
There are several different types of nuclear reactors, but they all share some common characteristics. They all have a supply of radioactive fuel pellets - usually uranium oxide - which are arranged in tubes to form fuel rods in active zonesereactor.

The reactor also has the previously mentioned managerserodAnd- made of a neutron-absorbing material such as cadmium, hafnium or boron, which is inserted to control or stop a reaction.
The reactor also has moderator, a substance that slows down neutrons and helps control the fission process. Most reactors in the United States use ordinary water, but reactors in other countries sometimes use graphite, or heavywowwaterat, in which hydrogen is replaced by deuterium, an isotope of hydrogen with one proton and one neutron. Another important part of the system is coolingand Iliquidb, usually ordinary water, which absorbs and transfers heat from the reactor to create steam to spin the turbine and cools the reactor area so that it does not reach the temperature at which the uranium will melt (about 3815 degrees Celsius).
Finally, the reactor is enclosed in shellsat, a large, heavy structure, usually several meters thick, made of steel and concrete that keeps radioactive gases and liquids inside where they can't harm anyone.

There are a number of different reactor designs in use, but one of the most common is pressurized water power reactor (VVER). In such a reactor, water is forced into contact with the core and then remains there under such pressure that it cannot turn into steam. This water then comes into contact with unpressurized water in the steam generator, which turns into steam, which rotates the turbines. There is also a design high-power channel-type reactor (RBMK) with one water circuit and fast neutron reactor with two sodium and one water circuits.
How safe is a nuclear reactor?
Answering this question is quite difficult and depends on who you ask and how you define “safe”. Are you concerned about radiation or radioactive waste generated in reactors? Or are you more worried about the possibility of a catastrophic accident? What degree of risk do you consider an acceptable trade-off for the benefits of nuclear power? And to what extent do you trust the government and nuclear energy?
"Radiation" is a strong argument, mainly because we all know that large doses of radiation, such as from a nuclear bomb, can kill many thousands of people.
Proponents of nuclear power, however, point out that we are all regularly exposed to radiation from a variety of sources, including cosmic rays and natural radiation emitted by the Earth. The average annual radiation dose is about 6.2 millisieverts (mSv), half of it from natural sources and half from man-made sources ranging from chest X-rays, smoke detectors and luminous watch dials. How much radiation do we get from nuclear reactors? Only a tiny fraction of a percent of our typical annual exposure is 0.0001 mSv.
While all nuclear plants inevitably leak small amounts of radiation, regulatory commissions hold plant operators to stringent requirements. They cannot expose people living around the plant to more than 1 mSv of radiation per year, and workers at the plant have a threshold of 50 mSv per year. That may seem like a lot, but according to the Nuclear Regulatory Commission, there is no medical evidence that annual radiation doses below 100 mSv pose any risks to human health.
But it's important to note that not everyone agrees with this complacent assessment of radiation risks. For example, Physicians for Social Responsibility, a longtime critic of the nuclear industry, studied children living around German nuclear power plants. The study found that people living within 5 km of plants had double the risk of contracting leukemia compared to those living further from nuclear power plants.
Nuclear reactor waste
Nuclear power is touted by its proponents as "clean" energy because the reactor does not emit large amounts of greenhouse gases into the atmosphere compared to coal-fired power plants. But critics point to another environmental problem: nuclear waste disposal. Some of the spent fuel from the reactors still releases radioactivity. Other unnecessary material that should be saved is high level radioactive waste, a liquid residue from the reprocessing of spent fuel, in which some of the uranium remains. Right now, most of this waste is stored locally at nuclear power plants in ponds of water, which absorb some of the remaining heat produced by the spent fuel and help shield workers from radiation exposure.
One of the problems with spent nuclear fuel is that it has been altered by the fission process. When large uranium atoms are split, they create byproducts—radioactive isotopes of several light elements such as Cesium-137 and Strontium-90, called fission products. They are hot and highly radioactive, but eventually, over a period of 30 years, they decay into less dangerous forms. This period is called for them Pperiodohmhalf-life. Other radioactive elements will have different half-lives. In addition, some uranium atoms also capture neutrons, forming heavier elements such as Plutonium. These transuranium elements do not create as much heat or penetrating radiation as fission products, but they take much longer to decay. Plutonium-239, for example, has a half-life of 24,000 years.
These radioactiveewastes high level of reactors are dangerous to humans and other life forms because they can release huge, lethal doses of radiation even from a short exposure. Ten years after removing the remaining fuel from a reactor, for example, they are emitting 200 times more radioactivity per hour than it would take to kill a person. And if waste ends up in groundwater or rivers, it can enter the food chain and endanger large numbers of people.
Because waste is so dangerous, many people are in a difficult situation. 60,000 tons of waste are located at nuclear power plants close to major cities. But finding a safe place to store waste is not easy.
What can go wrong with a nuclear reactor?
With government regulators looking back on their experience, engineers have spent a lot of time over the years designing reactors for optimal safety. It's just that they don't break down, work properly, and have backup safety measures if something doesn't go according to plan. As a result, year after year, nuclear power plants appear to be fairly safe compared to, say, air travel, which regularly kills between 500 and 1,100 people a year worldwide.
However, nuclear reactors suffer major breakdowns. On the International Nuclear Event Scale, which rates reactor accidents from 1 to 7, there have been five accidents since 1957 that rate from 5 to 7.
The worst nightmare is a cooling system failure, which leads to overheating of the fuel. The fuel turns to liquid and then burns through the containment, releasing radioactive radiation. In 1979, Unit 2 at the Three Mile Island nuclear power plant (USA) was on the verge of this scenario. Fortunately, a well-designed containment system was strong enough to stop the radiation from escaping.

The USSR was less fortunate. A severe nuclear accident occurred in April 1986 at the 4th power unit at the Chernobyl nuclear power plant. This was caused by a combination of system failures, design flaws and poorly trained personnel. During a routine test, the reaction suddenly intensified and the control rods jammed, preventing an emergency shutdown. The sudden buildup of steam caused two thermal explosions, throwing the reactor's graphite moderator into the air. In the absence of anything to cool the reactor fuel rods, they began to overheat and completely collapse, as a result of which the fuel took on a liquid form. Many station workers and accident liquidators died. A large amount of radiation spread over an area of 323,749 square kilometers. The number of deaths caused by radiation is still unclear, but the World Health Organization says it may have caused 9,000 cancer deaths.
Nuclear reactor manufacturers provide guarantees based on probabilistic assessmente, in which they try to balance the potential harm of an event with the likelihood with which it actually occurs. But some critics say they should prepare instead for rare, unexpected but highly dangerous events. A case in point is the March 2011 accident at the Fukushima 1 nuclear power plant in Japan. The station was reportedly designed to withstand a strong earthquake, but not one as catastrophic as the 9.0 magnitude quake that sent a 14-meter tsunami wave above dikes designed to withstand a 5.4-meter wave. The onslaught of the tsunami destroyed the backup diesel generators that were intended to power the cooling system of the plant's six reactors in the event of a power outage. So even after the Fukushima reactors' control rods stopped fission, the still-hot fuel allowed temperatures to rise dangerously inside the destroyed ones. reactors.

Japanese officials resorted to a last resort - flooding the reactors with a huge amount of sea water with the addition of boric acid, which was able to prevent a disaster, but destroyed the reactor equipment. Eventually, with the help of fire trucks and barges, the Japanese were able to pump fresh water into the reactors. But by then, monitoring had already shown alarming levels of radiation in the surrounding land and water. In one village 40 km from the plant, the radioactive element Cesium-137 was found at levels much higher than after the Chernobyl disaster, raising doubts about the possibility of human habitation in the area.